|
Anderson, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32-46.
|
Ando, H., Fujii, C., Kawanabe, M., Ao, Y., Inoue, T., Takenaka, A., 2018. Evaluation of plant contamination in metabarcoding diet analysis of a herbivore. Sci. Rep. 8, 15563.
|
Birdlife International, 2016. Lophophorus lhuysii. The IUCN Red List of Threatened Species 2016: e. T22679192A92806697. . (Accessed 04 September 2020).
|
Callahan, B.J., Mcmurdie, P.J., Holmes, S.P., 2017. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11, 2639-2643.
|
Chen, D., Li, C., Feng, L., Zhang, Z., Zhang, H., Cheng, G., et al., 2018. Analysis of the influence of living environment and age on vaginal fungal microbiome in giant pandas ( Ailuropoda melanoleuca) by high throughput sequencing. Microb. Pathogen. 115, 280-286.
|
Chen, Y.H., 2013. Conservation priority for endemic birds of mainland China based on a phylogenetic framework. Chinese Birds 4, 248-253.
|
Chua, P.Y.S., Lammers, Y., Menoni, E., Ekrem, T., Bohmann, K., Boessenkool, S., et al., 2021. Molecular dietary analyses of western capercaillies ( Tetrao urogallus) reveal a diverse diet. Environ. DNA 3, 1156-1171.
|
Chung, C.T., Wong, H.S., Kwok, M.L., Meng, Q., Chan, K.M., 2021. Dietary analysis of the House Swift ( Apus nipalensis) in Hong Kong using prey DNA in faecal samples. Avian Res. 12, 5.
|
CITES, 2016. Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) Appendices I, II and III. . (Accessed 04 September 2020).
|
Deagle, B.E., Thomas, A.C., McInnes, J.C., Clarke, L.J., Vesterinen, E.J., Clare, E.L., et al., 2019. Counting with DNA in metabarcoding studies: how should we convert sequence reads to dietary data? Mol. Ecol. 28, 391-406.
|
Fahner, N.A., Shokralla, S., Baird, D.J., Hajibabaei, M., 2016. Large-scale monitoring of plants through environmental DNA metabarcoding of soil: recovery, resolution, and annotation of four DNA markers. PLoS ONE 11, e0157505.
|
Forin-Wiart, M-A., Poulle, M-L., Piry, S., Cosson, J-F., Larose, C., Galan, M., 2018. Evaluating metabarcoding to analyse diet composition of species foraging in anthropogenic landscapes using Ion Torrent and Illumina sequencing. Sci. Rep. 8, 17091.
|
Gainsbury, A., Meiri, S., 2017. The latitudinal diversity gradient and interspecific competition: no global relationship between lizard dietary niche breadth and species richness. Global Ecol. Biogeogr. 26, 563-572.
|
Gao, Z.T., Wang, X.Y., Liu, Y., Wei, X.M., Han, J.P., Chen, S.L., 2018. Identification of Chinese patent medicines containing Fritillariae cirrhosae bulbus using ITS2 region. Sci. Sin. Vitae 48, 482-489. (in Chinese).
|
|
Gotelli, N.J., Colwell, R.K., 2011. Estimating species richness. In: Magurran, A.E., McGill, B.J. (Eds.), Frontiers in Measuring Biodiversity. Oxford University Press, New York, pp. 39-54.
|
Gul, M.R., Griffen, B.D., 2020. Diet, energy storage, and reproductive condition in a bioindicator species across beaches with different levels of human disturbance. Ecol. Indic. 117, 106636.
|
|
Guo, L., Liu, F.Q., Shi, L., Wen, L.Y., 2020. Physiological and ecological adaptability of Blood Pheasant (Ithaginis cruentus) and its favorite Bryophyte. Chin. J. Appl. Environ. Biol. 26, 1400-1405. (in Chinese).
|
Hacker, C.E., Hoenig, B.D., Wu, L., Cong, W., Yu, J., Dai, Y., et al., 2021. Use of DNA metabarcoding of bird pellets in understanding raptor diet on the Qinghai-Tibetan Plateau of China. Avian Res. 12, 42.
|
|
He, F.Q., Lu, T.C., 1985. Ecology of the Chinese Monal in winter. Zool. Res. 6, 345-352. (in Chinese).
|
|
He, F.Q., Lu, T.C., Lu, C.L., Cui, X.Z., 1986. Study on the breeding ecology of the Chinese Monal. Acta Ecol. Sin. 6, 186-192. (in Chinese).
|
Hsieh, T.C., Ma, K.H., Chao, A., 2016. iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Method. Ecol. Evol. 7, 1451-1456.
|
IUCN, 2020. The IUCN red list of threatened species. Version 2020-2. . (Accessed 04 September 2020).
|
Jayawardena, R.S., Purahong, W., Zhang, W., Wubet, T., Li, X., Liu, M., et al., 2018. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 90, 1-84.
|
Jia, H.Y., Liu, X.Q., Tian, N.F., Zhang, R., Wang, R.R., Liu, L.X., et al., 2021. Spring and summer diet composition of Tibetan Snowcocks. Chinese J. Ecol. 40, 470-479. (in Chinese).
|
Kartzinel, T.R., Chen, P.A., Coverdale, T.C., Erickson, D.L., Kress, W.J., Kuzmina, M.L., et al., 2015. DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proc. Nat. Acad. Sci. 112, 8019-8024.
|
Kluen, E., Nousiainen, R., Lehikoinen, A., 2017. Breeding phenological response to spring weather conditions in common Finnish birds: resident species respond stronger than migratory species. J. Avian Biol. 48, 611-619.
|
|
Long, T.L., Shao, K., Guo, G., Cheng, C.Y., Zou, X.Y., Landel, H., et al., 1998. Field tracking and ecological observation of the Chinese Monal in winter. Sichuan J. Zool. 17, 104-105. (in Chinese).
|
|
Lu, T.C., Liu, R.S., He, F.Q., Lu, C.L., 1986. Ecological studies on Chinese Monal. Acta Zool. Sin. 32, 273-279. (in Chinese).
|
|
Luo, K., Ma, P., Yao, H., Song, J., Chen, K., Liu, Y., 2012a. Molecular identification of Fritillariae cirrhosae Bulbus and its adulterants. World Sci. Technol. Modern. Tradit. Chin. Med. Materia Medica 14, 1153-1158. (in Chinese).
|
|
Luo, X., Wu, T.P., Huang, A.Q., 2016. Diet analysis and foraging strategy of two sympatric pheasants at Mt. Gaoligong in winter. Chin. J. Ecol. 35, 1003-1008. (in Chinese).
|
|
Luo, X., Xu, M.Y., Wu, L.X., 2012b. Spring diets and food nutrients of Sclater's Monal at Mt. Gaoligong, China. Sichuan J. Zool. 31, 17-22. (in Chinese).
|
|
Ma, G.Y., 1988. Observation on the Chinese Monal in Gansu Province. Sichuan J. Zool. 7, 41-42. (in Chinese).
|
Martin, K., Hik, D., 1992. Willow Ptarmigan chicks consume moss sporophyte capsules. J. Field Ornithol. 63, 355-358.
|
|
McClenaghan, B., Nol, E., Kerr, K.C.R., 2019. DNA metabarcoding reveals the broad and flexible diet of a declining aerial insectivore. Auk 136, 1-11.
|
McInnes, J.C., Alderman, R., Deagle, B.E., Lea, M-A., Raymond, B., Jarman, S.N., 2017. Optimised scat collection protocols for dietary DNA metabarcoding in vertebrates. Method. Ecol. Evol. 8, 192-202.
|
Moorhouse-Gann, R.J., Dunn, J.C., De Vere, N., Goder, M., Cole, N., Hipperson, H., et al., 2018. New universal ITS2 primers for high-resolution herbivory analyses using DNA metabarcoding in both tropical and temperate zones. Sci. Rep. 8, 8542.
|
National Forestry and Grassland Administration, National Development and Reform Commission, 2021. The outline of the national 14th Five-Year Plan on development of forestry and grassland conservation. . (in Chinese).
|
Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., et al., 2020. vegan: community ecology package. R package version 2.5-7. .
|
Pompanon, F., Deagle, B.E., Symondson, W.O.C., Brown, D.S., Jarman, S.N., Taberlet, P., 2012. Who is eating what: diet assessment using next generation sequencing. Mol. Ecol. 21, 1931-1950.
|
Prins, H.H.T., 1982. Why are mosses eaten in cold environments only? Oikos 38, 374-380.
|
R Core Team, 2021. R: a language and environment for statistical computing. .
|
Rognes, T., Flouri, T., Nichols, B., Quince, C., Mahe, F., 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584.
|
Shao, X.N., Lu, Q., Xiong, M.Y., Bu, H.L., Shi, X.Y., Wang, D.J., et al., 2021. Prey partitioning and livestock consumption in the world's richest large carnivore assemblage. Curr. Biol. 31, 4887-4897.
|
Shutt, J.D., Trivedi, U.H., Nicholls, J.A., 2021. Faecal metabarcoding reveals pervasive long-distance impacts of garden bird feeding. Proc. R. Soc. B. Biol. 288, 20210480.
|
|
Teng, L.W., Li, Y.X., Liu, Z.S., Dong, J.P., Yao, Z.C., Zhang, J.T., 2013. Summer diet composition of feral yak in Helan Mountains. J. Econ. Anim. 17, 192-196. (in Chinese).
|
|
Teng, L.W., Wang, Y., Li, L.Y., Yao, Z.C., Lu, P.F., Li, Y.X., et al., 2014. Autumn diet composition of feral yak (Bos grunnieus) in Helan Mountains. J. Anhui Agr. Sci. 42, 6258-6260. (in Chinese).
|
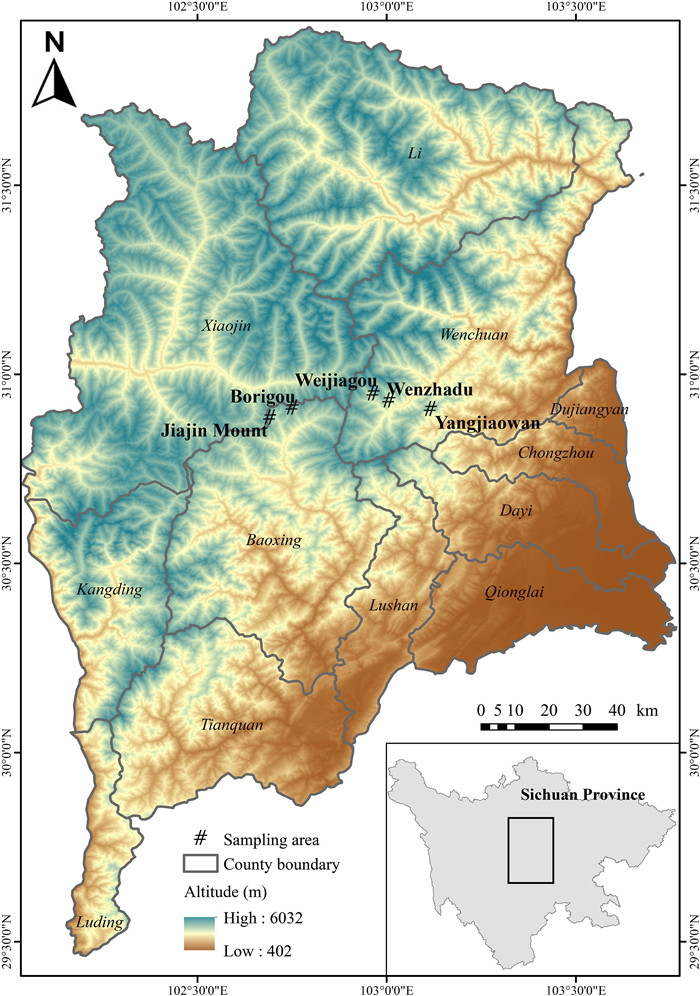
Wang, B., Xu, Y., Ran, J.H., 2017. Predicting suitable habitat of the Chinese Monal ( Lophophorus lhuysii) using ecological niche modeling in the Qionglai Mountains, China. PeerJ 5, e3477.
|
Ward, E.J., Levin, P.S., Lance, M.M., Jeffries, S.J., Acevedogutierrez, A., 2012. Integrating diet and movement data to identify hot spots of predation risk and areas of conservation concern for endangered species. Conserv. Lett. 5, 37-47.
|
|
Wickham, H., 2009. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York.
|
|
Xu, L.X., Wu, W., Ma, S.G., Ma, J., Cheng, K., 2015. Winter food habits of Hazal Grouse (Bonasa bonasia) in Liangshui National Nature Reserve. Chinese J. Wildl. 36, 402-406. (in Chinese).
|
Xu, Y., Wang, B., Zhong, X., Yang, B., Zhang, J.D., Zhao, C., et al., 2020. Predicting range shifts of the Chinese Monal ( Lophophorus lhuysii) under climate change: Implications for long-term conservation. Global Ecol. Conserv. 22, e01018.
|
|
Yao, Z.C., Liu, Z.S., Wang, Z.D., Hu, T.H., Li, Z.G., 2011. Winter and spring diet composition of feral yak in Helan Mountains, China. Acta Ecol. Sin. 31, 673-679. (in Chinese).
|
|
Yu, X., Chen, J.C., Wang, B., Yan, Y., Ran, J.H., He, F., et al., 2017. Population density estimation and habitat suitability assessment of Lophophorus lhuysii during breeding season in Xiaozhaizigou National Nature Reserve, Sichuan Province. Sichuan J. Zool. 36, 361-367. (in Chinese).
|
|
Zhang, B.W., Li, M., Ma, L.C., Wei, F.W., 2006. A widely applicable protocol for DNA isolation from fecal samples. Biochem. Genet. 44, 503-512.
|
|
Zhang, T., 1995. Distribution and ecology of Chinese Monals in Baishuijiang Nature Reserve, Gansu. Chinese J. Zool. 30, 25-28. (in Chinese).
|
Zhang, Z.W., Ding, C., Ding, P., Zheng, G., 2003. The current status and a conservation strategy for species of Galliformes in China. Biodivers. Sci. 11, 414-421.
|
|
Zheng, G.M., 2015. Pheasants in China. Higher Education Press, Beijing.
|
|
Zheng, H., Deng, K.Y., Chen, A.Q., Fu, S.B., Zhou, D., Wang, W.W., et al., 2019. Molecular identification and genetic relationship of Fritillaria cirrhosa and related species based on DNA barcode. Acta Pharm. Sin. 54, 2326-2334. (in Chinese).
|



 DownLoad:
DownLoad:











 Email Alerts
Email Alerts RSS Feeds
RSS Feeds