| Citation: | Tao Liu, Yongtao Xu, Canwei Xia, David Edwards, Xiaolong Hu, Yingyu Su, Jinsheng Xie, Weiwei Zhang. 2022: Multiple lines of evidence confirm that the critically endangered Blue-crowned Laughingthrush (Garrulax courtoisi) is an independent species. Avian Research, 13(1): 100022. DOI: 10.1016/j.avrs.2022.100022 |
The taxonomy of the Blue-crowned Laughingthrush (Garrulax courtoisi) and its relationship with the Yellow-throated Laughingthrush (G. galbanus) and G. c. simaoensis, a range-restricted subspecies in China, has not been fully elucidated. So the taxonomic status and system evolution of the three taxa G. courtoisi, G. galbanus and G. c. simaoensis need to be reclarified. Two gene sequences myoglobin (MYO) and the mitochondrial cytochrome coxidase subunit I (COI) were combined to investigate the phylogenetic relationships among courtoisi, simaoensis and galbanus, genetic data, combining with morphological, ecological and acoustic data were used to comb out the classification status and divergence level of the three taxa. Significant genetic and morphological differentiations (body size and plumage coloration) were detected between courtoisi and galbanus. However, no notable and reliable differences between the courtoisi and simaoensis were detected. The courtoisi, simaoensis and galbanus are clearly isolated in geographical distribution as a result of differing altitudes, climate conditions and habitats. The courtoisi has characteristic preference for nest location compared with galbanus. In addition, the results of song analysis also indicated that there are differences in maximum frequency between courtoisi and galbanus. G. courtoisi was confirmed to be an independent species based on genetic, morphological, geographical, ecological and vocal characteristics, and the validity of simaoensis as a subspecies still need more evidence. This study further confirmed the high conservation value of Blue-crowned Laughingthrush. In addition, due to the genetic differences between Simao and Wuyuan populations, this should be fully considered in future protection strategies.
The Blue-crowned Laughingthrush (Garrulax courtoisi) was listed as critically endangered (CR) by the IUCN due to its narrow distribution and small population size, with current breeding sites restricted to Wuyuan, Jiangxi Province, China and about 240 individuals left (BirdLife International, 2018). Effective and timely conservation efforts are sorely needed for the continuation of the species in perpetuity (Li et al., 2021). Its taxonomic status has been controversial since it was discovered in Wuyuan a century ago (Ménégaux, 1923; Berlioz, 1930; Zheng and Zheng, 1962; Long et al., 1994; Inskipp et al., 1996; Collar, 2006; He and Yang, 2006). The debate focused on the classification between this bird, the Yellow-throated Laughingthrush (G. galbanus), the other population of G. courtoisi distributed in Simao of Yunnan Province, China (G. c. simaoensis). Taxonomic status of the Blue-crowned Laughingthrush and its subspecies is directly related to its conservation value: with it being treated as an endangered monotypic species, a subspecies of an endangered species, or just an endangered geographical population of a least concern species.
The specific epithet G. courtoisi was suggested by Ménégaux (1923) based on two individuals collected from Wuyuan, Jiangxi Province, China, in 1919. Berlioz (1930) combined it with G. galbanus and named G. g. courtoisi. Several individuals resembling G. galbanus were first discovered in Shitou Mountain of Yunnan Province in 1956, and these birds were subsequently treated as a subspecies of G. galbanus and nominated as G. g. simaoensis in 1962 (Zheng and Zheng, 1962).
Long et al. (1994) suggested that G. galbanus should be treated as a monotypic species and separated from G. g. courtoisi and G. g. simaoensis. This view was supported by Inskipp et al. (1996), who noted the difference in morphology of galbanus, courtoisi and simaoensis. Collar (2006) compared the specimens of the galbanus and courtoisi, and proposing the courtoisi as a full species, and named Blue-crowned Laughingthrush. This view was accepted by most international ornithologists, many references treated the population distributed in Wuyuan as a full species and viewed the other population distributed in Yunnan as the subspecies of G. courtoisi, such as BirdLife International, the Howard and Moore Complete Checklist of the Birds of the World (4th edition) and the IOC World Bird List 12.1 (Gill et al., 2022).
Genetic evidence supporting that the Blue-crowned Laughingthrush as a full species was nevertheless insufficient (He and Yang, 2006). Furthermore, Long et al. (1994) noted a yellow-grey chest band in simaoensis that was not present in courtoisi; therefore, he suggested that this characteristic should be used to distinguish these two subspecies. However, this feature was also found on the individuals of the courtoisi (Wilkinson and He, 2010b). As a result, the rationality of simaoensis as a subspecies became dubious, and there are calls for it to be grouped with courtoisi (Collar and Robson, 2007).
Most traditional taxonomists define species based on morphological and ecological niches (Liu, 2016). However, the phylogenetic and cladistic species concepts based on DNA sequencing have been increasingly employed by avian researchers (Hebert et al., 2004). To clarify the confusing taxonomy of G. courtoisi and the phylogenetic relationships among the three geographical populations, we examined the genetic features of the courtoisi and galbanus, as well as the simaoensis, and we subsequently combined the phylogenetic results with the morphological, acoustical and zoogeographical characters of the birds to discuss the validity of courtoisi as a new species and simaoensis as a subspecies, as well as the evolutionary history of these populations, which can provide important information on the conservation strategy development according to the taxonomic status of this species.
Mist net was used to capture the G. c. courtoisi in breeding period in Wuyuan, and 5–20 μL of blood samples were collected. Morphological parameters including plumage colour (crown, occiput, throat, back, flank, wings, breast and belly, central tail-feathers, and outer tail-feathers) and body size (length of bill, wing, tarsus, and tail) were noted and measured respectively. All of the parameters were measured by a same person.
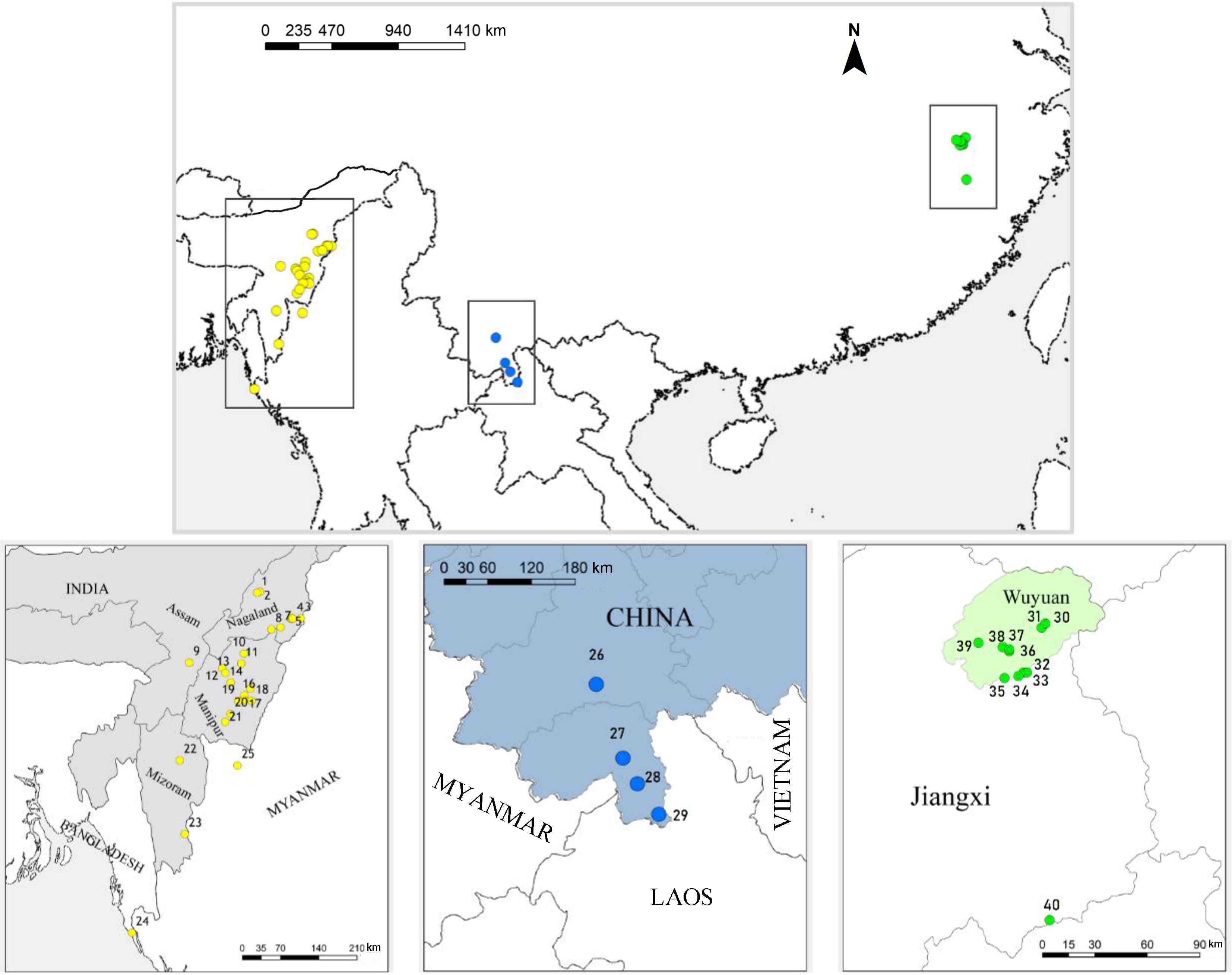
A total of 36 species in the Garrulax complex, 4 approximate outgroup species (Pomatorhinus ruficollis, Stachyris nigriceps, Alcippe morrisonia, and Pellorneum ruficeps) and 2 rooting outgroup species (Passer montanus and Parus major) were chosen in our study based on the results of Cai et al. (2019). Our dataset includes 56 samples, each taxon containing 1–3 samples (Appendix Table S1). We followed del Hoyo and Collar (2016) for our taxonomic classification.
The DNeasy Blood and Tissue Kit (Qiagen) was used to extract DNA from blood samples following the manufacturer's protocol. DNA of toepad samples from museum specimens was extracted following the methods of Irestedt et al. (2006, 2016). We amplified and sequenced one nuclear gene, myoglobin (MYO), and one mitochondrial gene, cytochrome oxidase subunit I (COI). Polymerase chain reaction (PCR) amplification of the blood samples was performed using published primers (Appendix Table S2) (Gelang et al., 2009; Cibois et al., 2018); for the toepad samples, six pairs of new primers (Appendix Table S2) were designed by primer-BLAST in GenBank (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index). Authenticity of the sequences obtained from toepad samples was tested following the methods of Alström et al. (2018). Published DNA sequences were downloaded from GenBank. Sequences from the same individual were chosen when possible (Appendix Table S1).
Sequences were aligned and checked using MEGAX (Kumar et al., 2018). To determine the best substitution evolutionary models (Appendix Table S3) implemented in the model-based analyses of phylogeny and divergence times, we used Partition Finder 2 (Lanfear et al., 2016) with Akaike's information criterion (AIC) being applied. Phylogeny was estimated by Bayesian inference (BI) using MrBayes 3.2.6 (Ronquist et al., 2012) and by maximum likelihood (ML) using IQ-TREE 1.6.9 (Nguyen et al., 2015).
Literatures regarding the morphological description or measurement of G. galbanus, G. c. courtoisi, and G. c. simaoensis were collected. Specimens of G. galbanus and G. c. simaoensis and some living individuals of G. c. courtoisi were also observed and measured, as few specimens of G. c. simaoensis were available. In addition to the measured individuals, we have also collected photos of these birds to supplement our qualitative morphology comparison. Principal component analysis (PCA) was performed on the body size parameters of G. galbanus, G. c. courtoisi, and G. c. simaoensis.
Information on the distribution (recorded sites, elevation, terrain, habitat, and climate) and breeding features (nest location, nest material, egg-laying period, clutch size, egg colour, and egg size) of G. galbanus, G. c. courtoisi, and G. c. simaoensis were obtained from the literature and our field investigation.
The sound recording files of G. galbanus and G. courtoisi were downloaded from xeno-canto.org. We used Avisoft-SASLab Pro 5.2.10 (Avisoft Bioacoustics, Berlin, Germany) to analyse the recordings. First, we used a high-pass filter at 1 kHz, which is lower than the frequencies of songs, to remove background noise (mostly caused by wind) and resampled the recordings at 11.025 kHz. Then, we created spectrograms with a fast Fourier transform length of 256 points, a hamming window with a frame size of 100% and an overlap of 50%, a frequency resolution of 43 Hz, and a time resolution of 11.6 ms. On the spectrograms, strophes were separated from each other by pauses (always more than 1 s) and organized by elements that are defined as a continuous trace on a spectrogram. A total of 19 strophes from 10 recordings (4 for G. galbanus and 6 for G. courtosis) were measured. The following 6 variables were measured for each strophe: minimum frequency, maximum frequency, peak frequency (the frequency associated with the maximum energy), duration, number of elements, and number of distinct elements.
The mitochondrial gene COI is relatively stable and is often selected as a standard DNA marker (Hajibabaei et al., 2006). Divergence time was estimated in BEAST 1.8.4 (Drummond et al., 2012) using the relaxed molecular clock model based on the full partition strategy with the best substitution model for each locus (Appendix Table S3). We implemented the best ML tree as a starting tree and applied the "birth-death" incomplete sampling specification tree prior. In addition, we also employed the substitution rate of mitochondrial gene COI (1.8% substitutions/site/million year) (Lavinia et al., 2016) with a relaxed clock model to estimate the divergence time. These analyses were run for 200 million generations in total and were sampled every 1000 generations. TRACER v1.7.1 (Rambaut et al., 2018) was used to examine the effective sample size (ESS above 200) to evaluate the convergence of MCMC chains. Finally, we discarded the first 20,000 trees as "burning" and used the remaining 100,000 trees to produce a maximum clade credibility (MCC) tree. The Fig Tree v1.4.4 was used to visualize the results (Rambaut, 2018) and we used the 95% height posterior density as standard deviation.
For the morphometric comparisons, if the data were normally distributed, t-test was used; if not, Kolmogorov-Smirnov test was used. In addition, the clustering relationship of the three taxa was judged by the comprehensive score of each parameter. We only compared the body size difference by employing a statistical test between G. galbanus and G. c. courtoisi. Data analyses were conducted in SPSS 17.0.
MANOVA was used to assess the overall differences between G. galbanus and G. courtoisi followed by an independent sample t-test for each variable. Discriminant function analysis (DFA) was used to determine whether the sounds from different taxa could be distinguished. The results from leave-one-out cross-validation are reported as percentages of recordings correctly assigned in DFA. In leave-one-out cross-validation, each recording was assigned to a taxon based on discriminant functions calculated from all recordings except the one being classified.
The data set consisted of 111 sequences given a total of 1355 loci from published data including 17 new sequences generated from this research (Appendix Tables S1 and S3). For the new sequences, the length of the COI gene ranged from 211 to 594 bp, and the length of the MYO gene ranged from 182 to 667 bp (Appendix Table S4).
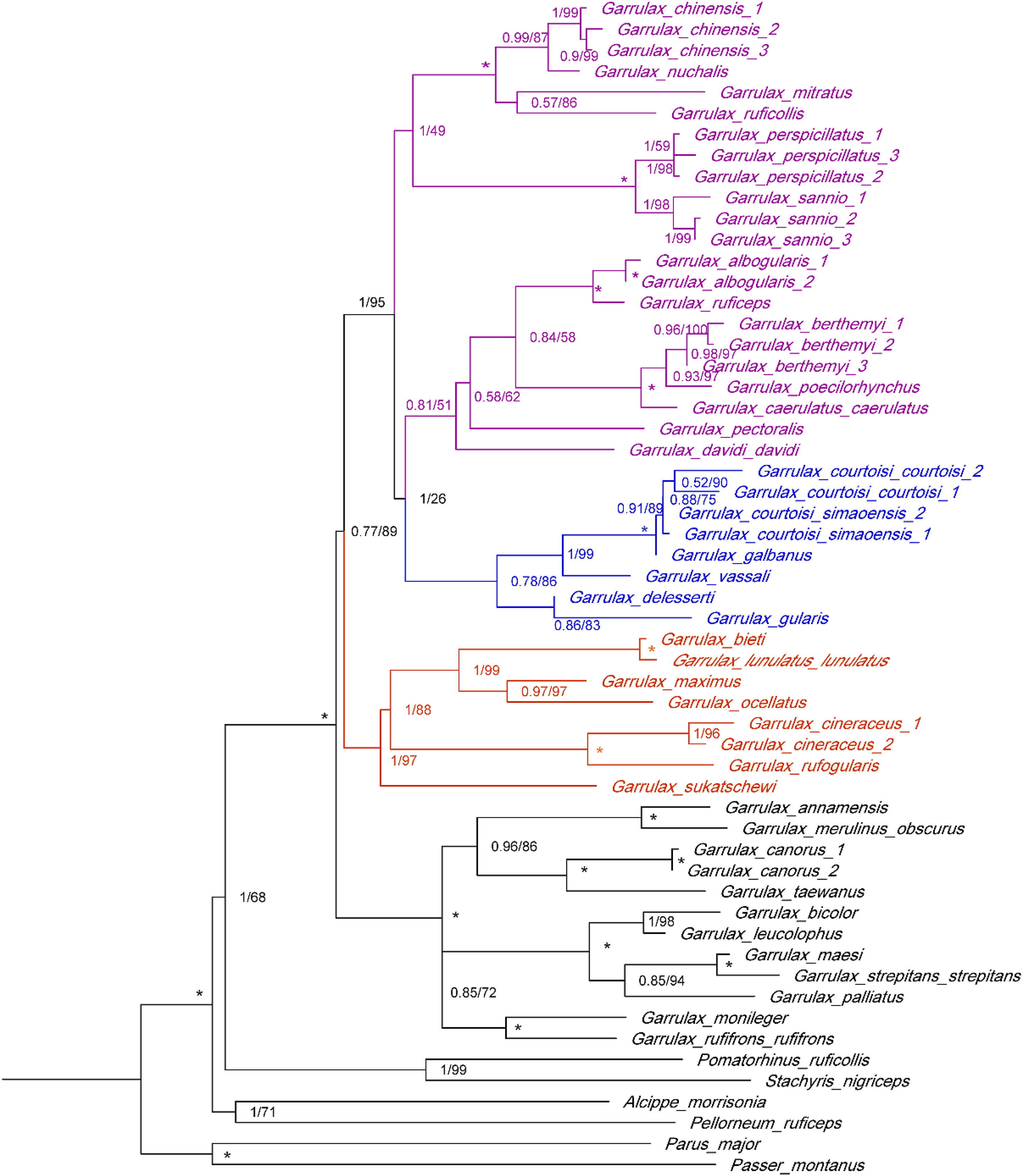
The topological structure of the ML tree rebuilt by IQ-TREE 1.6.9 was identical to that of the BI tree, and both of them had high node support rates (Fig. 1). The phylogenetic tree was established by integrating BI and ML trees. We identified G. c. courtoisi and G. c. simaoensis as two well-supported sister species, and they were sister branches with G. galbanus (Fig. 1).
According to the results of the BEAST analysis, the estimated divergence time between courtoisi and simaoensis (0.54 million years ago, Mya, range 0.22–0.96 Mya) suggested a relatively recent split compared with courtoisi and galbanus (0.74 Mya, range 0.34–1.29 Mya, approximately in the late Pleistocene) (Appendix Fig. S1).
The morphological descriptions of the courtoisi, simaoensis and galbanus were assessed (Appendix Table S5). The courtoisi's crown and occiput feather is dark blue while galbanus's is olive green. The edge of the primaries is blue in courtoisi, and it is grey in galbanus. In addition, the mask area of the courtoisi was larger than that of the galbanus (Collar, 2006) (shown in Appendix Fig. S2).
We noticed that there was a small blue naked patch behind the eyes in some photos of the galbanus. We proceeded to check the living individuals and specimens of courtoisi and simaoensis, none of which exhibited this feature.
Etchécopar and Hüe (1983) illustrated that courtoisi is yellow below with greyish-sullied flanks, and its underparts also yellow. We compared the breast and flank colours of simaoensis, courtoisi and galbanus by examining specimens and photos. The abdomen colour of simaoensis was a duller yellow than that of courtoisi, and the grey degree and area of the flank of simaoensis seem deeper and larger than courtoisi (Appendix Fig. S3).
The wing, tail, tarsus and bill of galbanus were all shorter than those of courtoisi and simaoensis, and all the body parameters of courtoisi were larger than those of simaoensis, except the tail (Table 1). There were significant differences in wing length (Z = 2.508; p < 0.0001), bill length (Z = 2.410; p < 0.0001), and tarsus length (Z = 2.932; p < 0.0001) between courtoisi and galbanus. The PCA results also showed clear differences between the courtoisi and galbanus, and the body size of simaoensis were between those of courtoisi and galbanus (Fig. 2).
| galbanus museum (Mean ± SE, n)# | simaoensis museum (Mean ± SE, n) (Cheng and Tang, 1982) | courtoisi living (Mean ± SE, n) | |
| Wing | 95.34 ± 0.38, 44b | 100.00 ± 2.08, 3 | 101.54 ± 0.82, 23a |
| Tail | 97.70 ± 0.67, 44a | 103.33 ± 2.03, 3 | 100.19 ± 0.87, 23a |
| Tarsus | 34.25 ± 0.23, 42c | 35.83 ± 0.44, 3 | 38.89 ± 0.63, 14d |
| Bill | 22.45 ± 0.18, 44e | 23.17 ± 0.60, 3 | 25.50 ± 0.74, 19f |
| # indicates data from 44 adult galbanus specimens which were kept at the Natural History Museum, Tring, U.K. The tarsus of two specimens were damaged, so the data were absent. courtoisi's data come from field measurement. Different lowercase letters indicate significant differences. | |||
The taxon galbanus is generally accepted to occur in Manipur in the northeast of India (Godwin-Austen, 1874; Hume, 1888; Baker, 1932; Long et al., 1994) and along the Naga Mountains in western Myanmar (Hopwood and Mackenzie, 1917) to Bangladesh (Thompson et al., 1993; Long et al., 1994) (Fig. 3). These sites were located in mountainous or low hilly areas at an altitude of approximately 610–1300 m, and the habitats were primarily high grass, secondary broad-leaved forests and shrubs (Collar and Robson, 2007). Records of galbanus in Nagaland were mostly made by bird photographers and birdwatchers (for information and sources, see Fig. 3 notes). Observations were rare in India.
The records of simaoensis were notably rare. The earliest was at Shitou Mountain, Simao, Yunnan, where the type specimens were collected (Cheng and Tang, 1982). However, there were almost no records made thereafter (Wilkinson and He, 2010b; He et al., 2017), except a documented record in the forest of the hill-valley zone of Menglun, Nangong Mountain and Longmen, Yunnan, in 2000 (Wang et al., 2000).
The breeding sites of courtoisi are better known; however, the wintering area of this bird has not been characterized. One sighting was in the north of Wuyi Mountain in the nonbreeding season (Cheng and Lin, 2011).
The three populations are approximately 1800–2100 km apart from each other (Fig. 3), with three locations belonging to subtropical or tropical monsoon regions with warm and wet weather. Out of the three locations, temperature in Wuyuan varied the greatest, while rainfall was highest in Manipur and lowest in Xishuangbanna (Table 2).
| galbanus | simaoensis | courtoisi | |
| Locations | India: Assam, Nagaland, Manipur, Mizoram; Bangladesh; Myanmar: Chin Hills (Long et al., 1994) | China: Simao, Yunnan (Cheng and Tang, 1982) | China: Wuyuan, Jiangxi (Hong et al., 2003) |
| Elevation (m) | 610–1300 (Collar and Robson, 2007) | 1340–1430 (He et al., 2007) | < 500; breeding ground ~ 100 (He et al., 2017) |
| Terrain | Hill (Collar and Robson, 2007) | Hill, valley (Cheng and Tang, 1982; Wang et al., 2000) | Hill, valley (He et al., 2017) |
| Habitat | Tall grass, mixed secondary forest and scrub (Collar and Robson, 2007) | Small broad-leaved trees, dense secondary forests only about 3 m in height; shrubs, tropical rainforest, woodland with large trees (Cheng and Tang, 1982; Wang et al., 2000; Wilkinson and He, 2010a) | Village fengshui forest, evergreen broadleaf forest, shrubs, cultivated land (Hong et al., 2003, 2006; Wilkinson et al., 2004) |
| Climate | Tropical monsoon climate, annual average rainfall of 2100 mm, annual maximum average temperature 24.7 ℃, annual minimum average temperature 12.8 ℃ (Singh et al., 2016) | Tropical monsoon climate with an average annual temperature of 15.1–21.7 ℃; the hottest month in June (average temperature 17.9–25.5 ℃), and the coldest month in January (average temperature 8.8–16 ℃); annual rainfall 1490 mm (Song et al., 2010; Zhu et al., 2015) |
Subtropical monsoon climate, warm and humid climate, with an average annual temperature of 16.7 ℃, the lowest average temperature in January of the coldest month being 0.4 ℃, the highest average temperature of July in the hottest month 34.2 ℃, and the average annual precipitation 1821 mm (Rao, 1996) |
The preferred elevation of the three groups was found to differ. The taxon galbanus preferred a medium elevation of about 610–1300 m (Collar and Robson, 2007); the preferred elevation for simaoensis was higher at about 1340–1430 m (He et al., 2007), however, the courtoisi was only found in low elevation areas, with the breeding sites at around 100 m (He et al., 2017).
It was found that galbanus nests in bushes or shrubs of 70–300 cm in height. The exterior component of the nest is roughly made of grass-stems with the ends left sticking out untidily in all directions and are lined with yellow grass seed stems.
There are often a few moss roots and small twigs mixed with the main structure of the nest (but not with the lining), and the interior component of the nest was made from thinner grass, twigs and seeds lined inside (Hopwood and Mackenzie, 1917; Baker, 1894). The yellow lining was thought to be the main feature of the galbanus. By contrast, courtoisi builds nests on big trees in small patch forests and isolated old trees in or near the villages along the river in low elevation areas of Wuyuan (Zhang et al., 2017; Liu et al., 2020), with the nest materials consisting mainly of thin vine, grass and palm silk (Huang et al., 2018; Table 3; Appendix Fig. S4). Breeding information of the simaoensis was lacking due to the difficulty in locating their breeding sites.
| Wild galbanus (Baker, 1932; Wilkinson and He, 2010b) | Captive simaoensis (Pasini et al., 1994) | Wild courtoisi (this study) | Captive courtoisi (Wilkinson and He, 2010b) | |
| Mean egg size (length × breadth) (mm) | 25.8 × 18.6 (n = 80) | 28.0 × 20.15 (n = 6) | 26.2 × 18.1 (n = 14)# | 26.0 × 18.4 (n = 89) |
| Egg size range (mm) | Length: 23.5–28; breadth: 16.5–20.1 | length: 27.3–29.0; breadth: 20.0–20.5 | Length: 24.1–29.0; breadth: 17.0–19.0 | Length: 18.8–29.9; breadth: 14.8–19.6 |
| Egg-laying period | April–June | ? | April–June | ? |
| Clutch size | 2–4 (4 is exceptional) | 3–5 | 3.08 ± 0.2 SE (min. = 1; max. = 5; n = 36) | ? |
| Egg colour | Usually white, but occasionally pale blue | White | White | White |
| Nest material | Grass-stems, yellow grass seed stems, few moss roots and small twigs (Hopwood and Mackenzie, 1917) | ? | Yellow grass seed stems, few moss dark brown nest material (dried fine vines, grass and twigs) | ? |
| Nest location | Low bush or shrub (70–304.8 cm) (Hopwood and Mackenzie, 1917) |
? | Thick (diameter: 40–80 cm) and tall (> 20 m) trees (Huang et al., 2018) | ? |
| # indicates that two egg parameters come from literature Wilkinson and He (2010b); ? indicates lack of data. | ||||
It was also known that galbanus laid eggs between April and June, and the clutch size varied from 2 to 4, with the higher limit being a rare occurrence. Egg colour was usually white and occasionally light blue (Baker, 1932). The breeding period of courtoisi is similar, and the clutch size was 3.08 ± 0.2 (n = 36; this study); however, no colour beside white was observed on the eggs. There were no significant differences in the egg size of courtoisi and galbanus in the field; however, the egg size of the Blue-crowned Laughingthrush (unknown subspecies) in the captive environment was greater than that of courtoisi and galbanus in the wild (Table 3).
From literature, courtoisi is known to be a cooperative breeder (Wilkinson et al., 2004; Wilkinson and He, 2010b; He et al., 2017). Although Baker (1932) noted that the nests of galbanus were not far from each other, it was uncertain whether they were cooperative breeders (Wilkinson and He, 2010a).
Sound features are significantly different between taxa (MANOVA: Pillai's Trace = 0.96; F6, 12 = 51.82; P < 0.001). Specifically, galbanus has a relatively lower maximum frequency than courtoisi (Table 4). For the total 19 strophes (9 in galbanus, 10 in taxon courtoisi), DFA achieved 94.74% accuracy in distinguishing between the two taxa. Only one strophe from courtoisi was incorrectly assigned to galbanus.
| Variables | galbanus (mean ± SD) | courtosis (mean ± SD) | t17 | P |
| Minimum frequency (kHz) | 1.77 ± 0.06 | 1.79 ± 0.62 | −0.09 | 0.928 |
| Maximum frequency (kHz) | 2.77 ± 0.10 | 3.46 ± 0.69 | −2.93 | 0.009 |
| Peak frequency (kHz) | 2.31 ± 0.15 | 2.82 ± 0.73 | −2.05 | 0.056 |
| Duration (s) | 1.21 ± 0.31 | 1.63 ± 0.72 | −1.61 | 0.125 |
| Number of elements | 4.44 ± 1.13 | 7.00 ± 3.53 | −2.07 | 0.054 |
| Number of distinct elements | 3.00 ± 0.00 | 2.40 ± 1.71 | 1.05 | 0.309 |
Integrative taxonomy is commonly used nowadays for species classification. By using multiple lines of evidence from different traits that may contribute to speciation, a mostly similar interpretation will lend credence to the interpretation. In this study, we will be using morphology, ecology and genetics to delineate a full species using the morphospecies, ecospecies, and phylogenetic species concept (Hong, 2016). When species are in the early stage of differentiation, only a few factors for species could be satisfied, but not all (Liu, 2016).
Although the differentiation between G. courtoisi and G. galbanus occurs in the late Pleistocene, morphological differences between them are evident. First, their feather colours on the crown, neck and edges of the primaries are different; second, there is a blue bare patch behind the eyes of G. galbanus, but not G. courtoisi; and third, the lengths of the wing, tarsus and bill of G. courtoisi are greater than those of G. galbanus. However, no significant morphological differences were found between courtoisi and simaoensis. The laughingthrushes seldom migrate long distances due to their weak flying ability. The geographic isolation between the three units created conditions for differentiation, and the westward colonization of simaoensis might be blocked by the Hengduan Mountains in China.
These birds generally have black eye masks and yellowish throats and breasts and are mostly distributed in the Indian subcontinent, Myanmar, Thailand, Laos, northern Vietnam and southwestern China (MacKinnon et al., 2000). Hume (1888) proposed that there was a small patch of light blue pelt at the end of the black ear feather of the galbanus. This feature was significant in the wild; however, Godwin-Austen (1874) did not mention it, as this feature might not appear in the specimen. Ripley (1952) described the blue pelt patch behind the eyes of galbanus recorded on Naga Mountain, India. A photo of galbanus taken by Ramki Sreenivasan in east Nagaland, India, also clearly showed this feature (Wilkinson and He, 2010a; Appendix Fig. S2B); these records are all in congruence with the description of Hume (1888). Therefore, we considered the blue pelt patch behind eyes should be an assertion feature to distinguish courtoisi from galbanus (Wilkinson and He, 2010a), and the lack of blue pelt patch in Godwin-Austen (1874)'s description could easily be due to the foxing of the specimen.
Unfortunately, the specimens of simaoensis were collected in winter, and the differences in colour is likely to be related to season. Therefore, few stable differences in plumage colour were found between courtoisi and simaoensis.
In ecological niche, courtoisi breeds in trees along low river valleys with altitude approximately 100 m, while galbanus lives and breeds in bushes and shrubs on hills at higher altitudes of approximately 610–1300 m. The breeding of the courtoisi is notably dependent on small patches of forests in or beside the villages (Zhang et al., 2017), whereas galbanus lives far from human environments, and they inhabit notably different elevations and habitats. Such a division in breeding preference may serve as evidence for potential behavioural reproductive isolation between the two taxa.
In our study, the taxonomic status of G. courtoisi as a valid species was also supported by the genetic results, and the divergence time between G. courtoisi and G. galbanus (0.74 Mya) was earlier than other recent split species in Garrulax, such as G. bieti and G. lunulatus (0.42 Mya) (del Hoyo and Collar, 2016). Considering the phylogenetic affinity of these birds, it is reasonable to suspect that the reproductive isolation between G. courtoisi and G. galbanus is also incomplete. In addition, the divergence time of most species in Garrulax was 1–5 Mya (Luo et al., 2008) and most of them were considered diverging from the period of the late Pleistocene including galbanus, courtoisi, and simaoensis.
During the Pleistocene glacial and interglacial periods, the different groups of laughingthrush migrated to different refuges in the cold period from the original warm habitat and began to evolve separately, promoting intraspecific and interspecific differentiation (Hewitt, 2000, 2004). We infer that the common ancestors of galbanus, courtoisi, and simaoensis separated into three ethnic groups to explore the suitable habitats during the alternate ice ages. The first group migrated along the Naga Mountains to low-latitude areas that were warmer and more humid post-glacial, forming the population of G. galbanus distributed along the Naga River valley. The second group restricted to the Hengduan Mountains and withdrew to the southwest, forming the simaoensis. The last group migrated along the vast subtropical hilly valleys and then retreated to the north of the Wuyi Mountains, as the climate was warmer in low-altitude areas during that period, thereby forming the courtoisi. After experiencing adaptations to different climates and environments, the Simao and Wuyuan populations are distinct in morphology from the Naga population but not from each other.
Differences in vocalizations are thought to be particularly taxonomically informative in lineages in which song development is innate (e.g., the suboscine passerines) where vocal divergence reflects genetic divergence in these groups (Touchton et al., 2014). Edwards et al. (2016) recorded the songs of galbanus at Nagaland, north-eastern India, and compared them with the songs of courtoisi recorded in Wuyuan. Very distinct differences were found in the acoustics of courtoisi and galbanus, supporting the view that these taxa are accorded as species rank.
In 76% of birds, body size increases with latitude (Ashton, 2002) following Bergmann's rule (Bergmann, 1847). The body size of courtoisi was larger than that of galbanus, as the distribution latitude of courtoisi (28–29° N) was higher than that of galbanus (21–26° N). The wings of courtoisi were longer than those of galbanus and simaoensis, which might be a selected adaptation to improve their flying ability due to their tendencies to build nests on tall trees.
Based on the genetic, morphological, ecological and vocal results, we support the opinions of Long et al. (1994) and Collar (2006) that G. courtoisi should be treated as a valid species, and whether simaoensis should be treated as the subspecies of G. courtoisi still requires more evidence and further discussion. However, the differentiation in breeding behaviour and genetics that may provide preliminary support for simaoensis being a distinct taxon cannot be ignored. The retention of G. courtoisi as a distinct species also underscores the need for urgent conservation action. G. courtoisi has undergone range contraction due to habitat loss, while it is at risk from cagebird trapping (Pasini et al., 1994), resulting in it being classified as critically endangered by Birdlife International. We need to cooperate with the government to protect the breeding areas, stop construction activities to avoid disturbance to the habitat, and control the ecotourism impact on the Blue-crowned Laughingthrush.
For the G. c. courtoisi, at present, Wuyuan is the only confirmed population of this species, with an isolated small population with only about 300 individuals (He et al., 2017). This taxon breeds in groups with several to dozens of individuals at different sites which are separated from each other. A noticeable decrease in breeding sites has been recorded with more than half recorded sites having been temporarily or permanently removed due to human activities. These disturbance from human construction activities, bird photographers and bird watchers have affected the behaviours of the population in Wuyuan, and led to the abandonment or transfer of breeding sites (Zhang et al., 2017; He et al., 2017). This does not bode well for this population in the long run as these disturbances are unlikely to change without conservationist intervention.
For the G. c. simaoensis, there have been no records for a long time, and it is likely to be extinct in the wild. While the taxonomic status of this taxon is still in flux, the possibility of it being a distinct taxon is high, given that the main differentiation between it and courtoisi is in breeding behaviour and genetics. Conservation efforts to restore the population in Yunnan should be given more attention. As individuals raised in zoos in America and Europe are likely exported from Yunnan through illegal trade (Wilkinson and He, 2010b), genetic profiling of these individuals should be carried out to determine their taxon assignment. It is likely that these individuals in the zoos will be the only source for restoring the wild population of the G. c. simaoensis in the future.
Comprehensive genetics, morphology, ecology, and song data analysis support the species-level differentiation of the Blue-crowned Laughingthrush (G. courtoisi) and Yellow-throated Laughingthrush (G. galbanus). There is also genetic differentiation between the two geographical populations of the Blue-crowned Laughingthrush (G. c. courtoisi and G. c. simaoensis). Combining the evolution and geological history of the genus Garrulax, it is speculated that the differentiation and distribution pattern of the Blue-crowned Laughingthrush may be related to the glacial events between 0.2 and 0.78 Mya.
WZ conceived the study. TL, YS, JX, WZ, and XH collected the data with assistance from DE. TL, YX, CX, YS, JX and WZ performed the analyses. TL, YX, and WZ wrote the article with assistance from DE. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
We are grateful to Huiqin Huang, Jinze Shi and Zhenhua Wei for help with wild sample collection and Shimin Tang, Prof. Zhijun Ma and Prof. Xin Lu for help with sample collection. We thank Dezhi Zhang and Tianlong Cai for help with the data analysis and figure mapping. We are grateful for receiving permission to use the vocal data. Thanks go to Dr. Mengyue Wu of Singapore National University for language proofreading and writing. This study was supported by the National Natural Science Foundation of China (No. 31660608, 31360521).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100022.
|
Baker, E.C.S., 1894. The birds of North Cachar. Bombay Nat. Hist. Soc. 8, 162-211.
|
|
Baker, E.C.S., 1932. The Nidification of Birds of the Indian Empire, 1. Taylor and Francis, London.
|
|
Bergmann, C., 1847. About the relationships between heat conservation and body size of animals. Goett. Stud. 1, 595-708.
|
|
Berlioz, J., 1930. Révision systématique du genre Garrulax lesson. L' Oiseau 11, 1-9.
|
|
Cheng, S., Lin, J., 2011. A survey on avian diversity in Wuyishan National Nature Reserve, Jiangxi. Chin. J. Zool. 46, 66-78. (in Chinese).
|
|
Cheng, T., Tang, R., 1982. A new subspecies of Garrulax galbanus from Yunnan, China. Sinozoologica. 2, 1-2.
|
|
Collar, N.J., Robson, C., 2007. Family Timaliidae (babblers). In: del Hoyo, J., Elliott, A., Christie, D.A., (Eds. ), Handbook of the Birds of the World, 12. Lynx Edicions, Barcelona, pp. 70-291.
|
|
del Hoyo, J., Collar, N.J., 2016. HBW and BirdLife International Illustrated Checklist of the Birds of the World. Volume 2: Passerines. Lynx Edicions and BirdLife International, Barcelona.
|
|
Etchecopar, R.D., Hue, F., 1983. Les oiseaux de Chine, de Mongolie et de Coree: Passereaux. Editions du Pacifique, Papeete.
|
|
He, F.Q., Yang, L., 2006. The new discussion on the classification of the G. courtoisi. Chin. J. Zool. 41, 127.
|
|
Hebert, P.D.N., Stoeckle, M.Y., Zemlak, T.S., Francis, C.M., 2004. Identification of birds through DNA barcodes. PLoS Biol. 2, 1657-1663.
|
|
Hong, Y., He, F.Q., Roland, W., Melaille, D., Zheng, P., 2003. Little-known oriental bird Courtois's Laughingthrush Garrulax galbanus courtoisi. Orient. Bird Club 38, 35-39.
|
|
Hopwood, J.C., Mackenzie, J.M.D., 1917. A list of birds from the North Chin Hills. J. Bombay Nat. Hist. Soc. 25, 72-91.
|
|
Inskipp, T., Lindsey, N., Duckworth, W., 1996. An Annotated Checklist of the Birds of the Oriental Region. The Oriental Bird Club, Sandy.
|
|
Long, A., Crosby, M., Inskipp, T., 1994. A review of the taxonomic status of the Yellow-throated Laughingthrush Garrulax galbanus. Orient. Bird Club 19, 41-48.
|
|
MacKinnon, J., Phillipps, K., He, F.Q., 2000. A Field Guide to the Birds of China. Hunan Education Press, Changsha, China. (in Chinese).
|
|
Ménégaux, A., 1923. Description du Garrulax courtoisi, nov. sp. De la Chine. Bull. Mus. Hist. Nat. 29, 287-288.
|
|
Pasini, A., Arsuffi, D., Micali, G., Violani, C.G., 1994. Identification and captive breeding of the Yellow-throated Laughingthrush Garrulax galbanus simaoensis in northern Italy. Orient. Bird Club Bull. 19, 49-50.
|
|
Rao, P.C., 1996. Study on the woody plant flora in Wuyuan County. J. Nanjing Forest. Univ. 20, 84–87 (in Chinese).
|
|
Thompson, P.M., Harvey, W.G., Johnson, D.L., 1993. Recent notable bird records from Bangladesh. Forktail 9, 13-44.
|
|
Wang, Z., Chen, J., Deng, X., Bai, Z., Yang, Q., Liu, Y., 2000. Morus macroura and the wildlife who eat his berry in different mountain regions, Xishuangbanna, Yunnan J. Mount. Sci. 18, 267-271. (in Chinese).
|
|
Wilkinson, R., He, F.Q., 2010a. Yellow-throated Laughingthrush Garrulax galbanus and Blue-crowned Laughingthrush G. courtoisi new observations and interpretations on their taxonomy. BirdingASIA 14, 73-82.
|
|
Wilkinson, R., He, F.Q., 2010b. Conservation of Blue-crowned Laughingthrush Garrulax courtoisi in Wuyuan, Jiangxi, and the search for 'lost' populations in Yunnan and Guangxi, China. BirdingASIA 13, 100-105.
|
|
Wilkinson, R., He, F.Q., Gardner, L., 2004. A highly threatened bird-Chinese Yellow-throated Laughingthrushes in China and in zoos. Int. Zoo News 51, 456-469.
|
|
Zheng, Z., Zheng, B., 1962. On birds from Hsi-Shuan-Pan-Na area and vicinity in Yunnan Province. Ⅲ. Acta Zool. Sin. 14, 74-94. (in Chinese).
|
| galbanus museum (Mean ± SE, n)# | simaoensis museum (Mean ± SE, n) (Cheng and Tang, 1982) | courtoisi living (Mean ± SE, n) | |
| Wing | 95.34 ± 0.38, 44b | 100.00 ± 2.08, 3 | 101.54 ± 0.82, 23a |
| Tail | 97.70 ± 0.67, 44a | 103.33 ± 2.03, 3 | 100.19 ± 0.87, 23a |
| Tarsus | 34.25 ± 0.23, 42c | 35.83 ± 0.44, 3 | 38.89 ± 0.63, 14d |
| Bill | 22.45 ± 0.18, 44e | 23.17 ± 0.60, 3 | 25.50 ± 0.74, 19f |
| # indicates data from 44 adult galbanus specimens which were kept at the Natural History Museum, Tring, U.K. The tarsus of two specimens were damaged, so the data were absent. courtoisi's data come from field measurement. Different lowercase letters indicate significant differences. | |||
| galbanus | simaoensis | courtoisi | |
| Locations | India: Assam, Nagaland, Manipur, Mizoram; Bangladesh; Myanmar: Chin Hills (Long et al., 1994) | China: Simao, Yunnan (Cheng and Tang, 1982) | China: Wuyuan, Jiangxi (Hong et al., 2003) |
| Elevation (m) | 610–1300 (Collar and Robson, 2007) | 1340–1430 (He et al., 2007) | < 500; breeding ground ~ 100 (He et al., 2017) |
| Terrain | Hill (Collar and Robson, 2007) | Hill, valley (Cheng and Tang, 1982; Wang et al., 2000) | Hill, valley (He et al., 2017) |
| Habitat | Tall grass, mixed secondary forest and scrub (Collar and Robson, 2007) | Small broad-leaved trees, dense secondary forests only about 3 m in height; shrubs, tropical rainforest, woodland with large trees (Cheng and Tang, 1982; Wang et al., 2000; Wilkinson and He, 2010a) | Village fengshui forest, evergreen broadleaf forest, shrubs, cultivated land (Hong et al., 2003, 2006; Wilkinson et al., 2004) |
| Climate | Tropical monsoon climate, annual average rainfall of 2100 mm, annual maximum average temperature 24.7 ℃, annual minimum average temperature 12.8 ℃ (Singh et al., 2016) | Tropical monsoon climate with an average annual temperature of 15.1–21.7 ℃; the hottest month in June (average temperature 17.9–25.5 ℃), and the coldest month in January (average temperature 8.8–16 ℃); annual rainfall 1490 mm (Song et al., 2010; Zhu et al., 2015) |
Subtropical monsoon climate, warm and humid climate, with an average annual temperature of 16.7 ℃, the lowest average temperature in January of the coldest month being 0.4 ℃, the highest average temperature of July in the hottest month 34.2 ℃, and the average annual precipitation 1821 mm (Rao, 1996) |
| Wild galbanus (Baker, 1932; Wilkinson and He, 2010b) | Captive simaoensis (Pasini et al., 1994) | Wild courtoisi (this study) | Captive courtoisi (Wilkinson and He, 2010b) | |
| Mean egg size (length × breadth) (mm) | 25.8 × 18.6 (n = 80) | 28.0 × 20.15 (n = 6) | 26.2 × 18.1 (n = 14)# | 26.0 × 18.4 (n = 89) |
| Egg size range (mm) | Length: 23.5–28; breadth: 16.5–20.1 | length: 27.3–29.0; breadth: 20.0–20.5 | Length: 24.1–29.0; breadth: 17.0–19.0 | Length: 18.8–29.9; breadth: 14.8–19.6 |
| Egg-laying period | April–June | ? | April–June | ? |
| Clutch size | 2–4 (4 is exceptional) | 3–5 | 3.08 ± 0.2 SE (min. = 1; max. = 5; n = 36) | ? |
| Egg colour | Usually white, but occasionally pale blue | White | White | White |
| Nest material | Grass-stems, yellow grass seed stems, few moss roots and small twigs (Hopwood and Mackenzie, 1917) | ? | Yellow grass seed stems, few moss dark brown nest material (dried fine vines, grass and twigs) | ? |
| Nest location | Low bush or shrub (70–304.8 cm) (Hopwood and Mackenzie, 1917) |
? | Thick (diameter: 40–80 cm) and tall (> 20 m) trees (Huang et al., 2018) | ? |
| # indicates that two egg parameters come from literature Wilkinson and He (2010b); ? indicates lack of data. | ||||
| Variables | galbanus (mean ± SD) | courtosis (mean ± SD) | t17 | P |
| Minimum frequency (kHz) | 1.77 ± 0.06 | 1.79 ± 0.62 | −0.09 | 0.928 |
| Maximum frequency (kHz) | 2.77 ± 0.10 | 3.46 ± 0.69 | −2.93 | 0.009 |
| Peak frequency (kHz) | 2.31 ± 0.15 | 2.82 ± 0.73 | −2.05 | 0.056 |
| Duration (s) | 1.21 ± 0.31 | 1.63 ± 0.72 | −1.61 | 0.125 |
| Number of elements | 4.44 ± 1.13 | 7.00 ± 3.53 | −2.07 | 0.054 |
| Number of distinct elements | 3.00 ± 0.00 | 2.40 ± 1.71 | 1.05 | 0.309 |